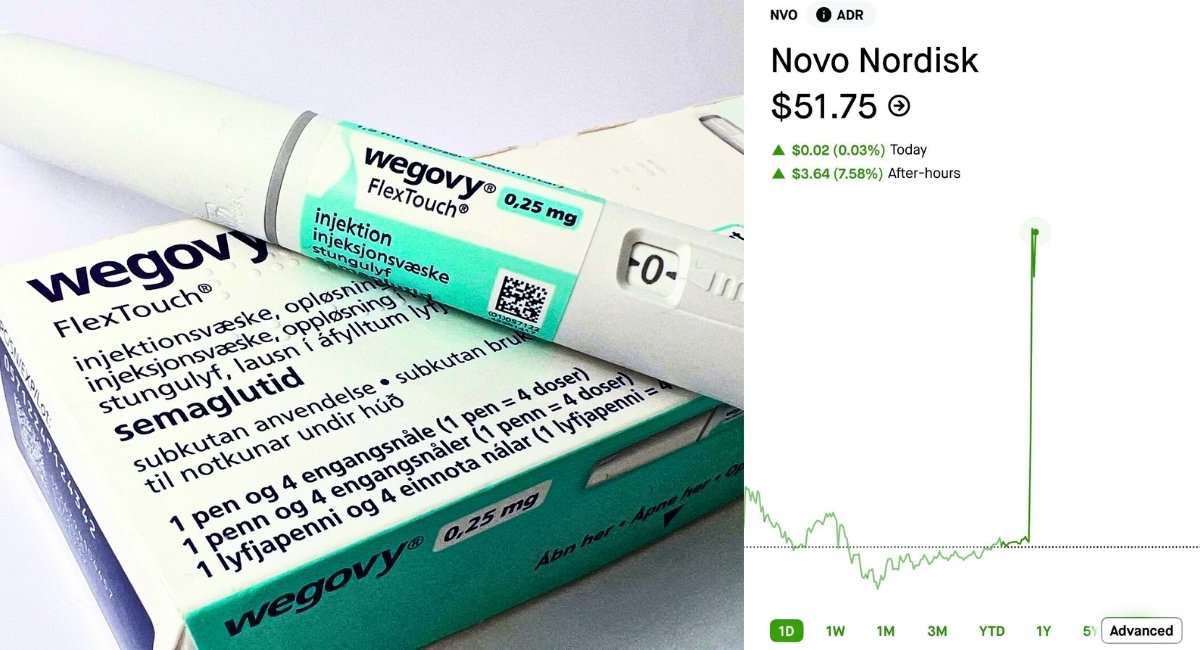
On December 22–23, 2025, the U.S. Food and Drug Administration (FDA) granted approval to Novo Nordisk’s Wegovy® oral pill, the first once-daily oral GLP-1 therapy for obesity, sending NVO stock sharply higher as the company prepares a full U.S. launch in early January 2026. This milestone — the first FDA-approved oral semaglutide for chronic weight management — not only expands access beyond injectable formulations but also positions Novo ahead in a booming obesity-treatment market.
Why This Approval Matters Now — And Why Investors Are Watching NVO Stock
In 2025, the biopharmaceutical landscape increasingly revolved around GLP-1 receptor agonists, the class of drugs that include semaglutide and tirzepatide. These medicines, originally developed for type 2 diabetes, have reshaped obesity care by producing substantial — and sustained — weight loss in clinical trials. Although injectable GLP-1 therapies dominated headlines and prescriptions, an effective oral alternative had remained elusive — until now.
Novo Nordisk’s FDA nod for the Wegovy pill changes that narrative. With the pill approved for adults with obesity (or overweight with related medical conditions) and shown to significantly reduce weight — and potentially cardiovascular risk — the drug re-positions Novo for growth at a time when injectable sales had softened amid competition and pricing pressure.
What the FDA Approval Means — The Science and Impact
First Oral GLP-1 for Chronic Weight Management
The Wegovy oral pill is a 25 mg once-daily semaglutide tablet, the same active molecule as in Novo’s injectable Wegovy and other GLP-1 products, re-engineered for oral absorption. The FDA’s decision was grounded in data from the OASIS 4 trial, the Phase 3 study that showed:
- ~16.6 % average weight loss versus placebo over 64 weeks in adults with obesity or overweight and at least one comorbidity.
- Comparable results to once-weekly injectable Wegovy, a leading treatment in the class.
- A consistent safety and tolerability profile aligned with existing GLP-1 therapies.
This is not a minor tweak — it’s a fundamental expansion of treatment options, especially for patients reluctant to use injections or who struggle with adherence.
What the Label Covers
The Wegovy pill is approved:
- To reduce excess body weight and help maintain long-term weight reduction.
- To reduce risk of major adverse cardiovascular events (like heart attack or stroke) in adults with known cardiovascular disease and obesity.
- Alongside reduced-calorie diets and increased physical activity — a standard of care in weight management.
This dual focus (weight and cardiovascular risk) significantly broadens clinical utility and healthcare provider interest, compared with many previous therapies.
NVO Stock Reaction — Market Moves on Breakthrough News
When the FDA announcement hit in late trading on December 22 – 23, 2025, NVO stock jumped significantly in after-hours and extended trading sessions:
- Shares climbed nearly 8 % after hours, reflecting investor excitement over the approval and the anticipated revenue boost from the oral formulation.
- Other market reports noted the stock rising strongly on the day, buoyed by early positioning ahead of the expected January 2026 commercial launch.
From a strategic investor perspective, this approval acts like a catalyst — a clear near-term event that transforms long-term forecasts into actionable valuation inputs. Institutions and hedge funds alike watch these inflection points closely, because predictable revenue ramps often follow regulatory wins of this magnitude.
Broader Obesity Treatment Landscape — Why Oral Matters
Convenience Drives Adoption
Clinically, that injectables like Wegovy and rivals have changed obesity care is undisputed; from 2021 onward, these medications dramatically expanded patient engagement. But reliance on weekly injections has been a barrier for many patients, due to needle anxiety, logistics, or cost and insurance challenges.
The once-daily pill addresses those barriers head-on:
- No refrigeration needed, unlike many injectables.
- Simplified administration, increasing likelihood of patient adherence.
- Lower starting price points — as low as $149 per month in the U.S., compared to several-hundred-dollar cost of injectables.
These factors aren’t aesthetic — they materially affect uptake, payer coverage discussions, and long-term demand. Investors who’ve covered biotech for years will tell you that adherence drives revenue as much as clinical efficacy does.
Competitive Dynamics — Lilly and Others in the Race
Novo Nordisk doesn’t own the GLP-1 class alone. Eli Lilly’s oral candidate, orforglipron, remains under FDA review but has yet to receive clearance, meaning the Wegovy pill enjoys a first-to-market advantage. Analysts and market observers estimate Lilly’s pill could arrive in mid-2026, creating a competitive follow-on effect — but not before Novo has established strong commercial traction.
This window matters. In pharmaceuticals, being the first approved and marketed often translates to stronger coverage, formulary placement, and patient/provider familiarity — all of which support pricing power and market share. Investors see this edge as a tangible source of revenue growth and valuation support.
What Traders and Analysts Are Saying
Market commentary and broker reports (as reflected in trading and price action) highlight a few key themes:
1. Reversal narrative for Novo
Following a tough 2025 — where NVO stock lagged due to competitive pressure and slower injectable sales — this regulatory milestone helps shift the narrative back to innovation and leadership in obesity care.
2. Expanded addressable market
Experts project that oral GLP-1s could command up to 20 % of the overall market by 2030, as convenience and affordability pull in patients previously reluctant to start injectable therapy.
3. Broader partnerships
Notably, partnerships like the announced WeightWatchers tie-up to retail Wegovy pills post-launch add strategic distribution channels that were absent from earlier product lifecycles.
Short-term traders see approval as a re-rating event, while long-term investors are focused on how the launch translates into sustained earnings growth in 2026 and beyond.
Risks and Considerations for Investors
Even with this breakthrough, several risk factors remain:
- Insurance coverage debates: While list price may be lower, actual patient out-of-pocket costs and coverage by Medicare/Medicaid remain uncertain.
- Side effect profile: GLP-1 drugs can cause gastrointestinal issues (nausea, diarrhea), which may affect discontinuation rates.
- Competition: Lilly’s orforglipron and future rivals will erode primo market share over time, requiring Novo to maintain clinical differentiation and pricing power.
- Patent timelines: Global patent expirations could influence generic entry in certain markets over the long term.
These are not proxy concerns — they shape how payers and providers integrate new therapies into practice.
Conclusion: A Strategic Inflection for Novo Nordisk and NVO Stock
Novo Nordisk’s FDA approval of the Wegovy oral pill is more than a regulatory checkbox — it’s a paradigm shift for obesity treatment and a potential long-term earnings vector for NVO stock as it heads into 2026.
Editorial Outlook:
In early January, as the pill hits the U.S. market, watch for real-world uptake indicators, payer coverage announcements, and pricing dynamics. If the Wegovy pill sees strong adoption and early cardiovascular outcome data remains compelling, this could redefine Novo’s growth trajectory and reshape investor sentiment in a sector that’s rapidly maturing.